Pune, India | September 23, 2025
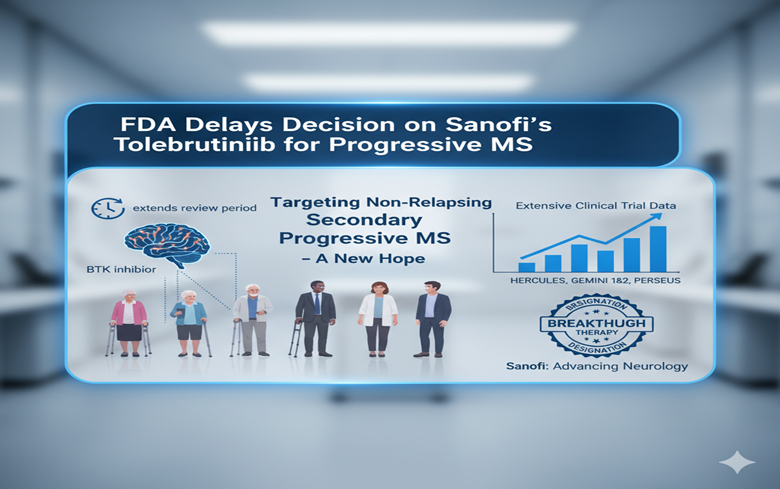
The U.S. Food and Drug Administration (FDA) has extended its review period for Sanofi’s oral Bruton’s Tyrosine Kinase (BTK) inhibitor, tolebrutinib, by three months. Sanofi is developing this drug to treat non-relapsing secondary progressive multiple sclerosis (nrSPMS)—a stage of MS marked by steady disability progression without relapses.
This delay follows Sanofi’s submission of additional analyses during the review process. The FDA classified the new data as a major amendment, which requires extra time for thorough evaluation.
Tolebrutinib targets a challenging phase of MS in which patients experience worsening symptoms despite the absence of relapses. The drug inhibits BTK, a key protein involved in immune cell signaling. Unlike many existing MS therapies, tolebrutinib crosses the blood-brain barrier and reduces inflammation directly in the central nervous system (CNS). Its mechanism aims to slow disability progression—a goal that current treatments struggle to achieve.
The FDA granted tolebrutinib Breakthrough Therapy designation for nrSPMS, reinforcing Sanofi’s confidence in its potential. This status highlights the drug’s ability to address critical unmet needs. Sanofi’s New Drug Application (NDA) includes data from the pivotal Phase III HERCULES trial, along with results from the GEMINI 1 and 2 studies, which focused on patients with relapsing MS. The ongoing PERSEUS trial is evaluating the drug’s effects in primary progressive MS, with results expected later this year.
The HERCULES trial enrolled patients with nrSPMS who had experienced disability progression over the past year and no relapses within the last two years. Researchers randomly assigned participants in a 2:1 ratio to receive either daily tolebrutinib or placebo for nearly four years. They measured confirmed disability progression over six months as the primary outcome. Secondary outcomes included MRI-based brain inflammation markers and tests of walking ability and upper limb function.
The GEMINI 1 and 2 studies compared tolebrutinib with teriflunomide in patients with relapsing MS. These trials focused on reducing relapse rates and slowing disease progression over three years. They also investigated how well tolebrutinib modulates immune response and preserves neurological function.
Sanofi emphasizes that tolebrutinib’s ability to penetrate the brain enables it to target both peripheral immune cells and CNS-resident microglia. Researchers believe these microglia drive the neuroinflammation that contributes to progressive disability in MS. While most approved therapies focus on peripheral B and T cells to manage relapses, they offer limited benefits once relapses stop. Tolebrutinib offers a new approach by addressing this gap in treatment.
The FDA extended the review period to thoroughly assess the newly submitted data. Sanofi confirmed its ongoing collaboration with the agency and pledged to provide any additional information required.
Patients with nrSPMS often struggle with symptoms such as impaired mobility, fatigue, and cognitive decline, all of which significantly reduce quality of life. Because most existing MS treatments do not adequately address this progressive phase, therapies like tolebrutinib are especially valuable.
Sanofi continues to pursue innovative treatments in its neurology portfolio. By combining its expertise in immunology and neurobiology with advanced technologies like artificial intelligence, the company aims to tackle complex neurological conditions involving neuroinflammation and neurodegeneration.
The FDA’s upcoming decision will hold major implications for patients, clinicians, and the broader MS community. If approved, tolebrutinib could become the first oral treatment specifically designed to slow disability progression in nrSPMS. This approval would mark a significant milestone in MS care.
Although the extended timeline delays approval, it reflects the FDA’s commitment to thorough evaluation. Sanofi remains focused on advancing tolebrutinib and believes the drug has the potential to improve outcomes for people living with progressive MS.
In conclusion, tolebrutinib stands out as a promising BTK inhibitor that targets CNS inflammation and may slow disability in nrSPMS. The FDA’s extended review reflects the complexity of evaluating such an innovative therapy. Approval would offer hope to patients who currently have few effective treatment options.